Probiotics: the latest gut-related discoveries herald a genuine revolution
 If we want to find unexplored ecosystems, we don’t need to trawl the depths of the ocean or scrutinise soil samples from some terrestrial planet. We simply have to look at our own intestines: more than 100,000 billion bacteria, which together weigh more than the brain, flourish in the human gut. Just a few decades ago, the existence of such a diverse microbiome would have been inconceivable. Indeed, we are still a long way from understanding the role of this ‘alien’ organ – which has 25 times more genes than exist in the human genome – but day by day, we’re discovering new ways in which it affects our general health. These surprising connections have revolutionised our understanding of some increasingly-prevalent diseases such as asthma, depression, obesity, allergies autism and auto-immune diseases.
If we want to find unexplored ecosystems, we don’t need to trawl the depths of the ocean or scrutinise soil samples from some terrestrial planet. We simply have to look at our own intestines: more than 100,000 billion bacteria, which together weigh more than the brain, flourish in the human gut. Just a few decades ago, the existence of such a diverse microbiome would have been inconceivable. Indeed, we are still a long way from understanding the role of this ‘alien’ organ – which has 25 times more genes than exist in the human genome – but day by day, we’re discovering new ways in which it affects our general health. These surprising connections have revolutionised our understanding of some increasingly-prevalent diseases such as asthma, depression, obesity, allergies autism and auto-immune diseases.
We are a ‘human-microbe’ hybrid
How is it that bacteria living in the gut can cause dysfunction that affects the whole body? Scientists are now convinced that it’s because humans and their microbiota exist in symbiosis. Over generations, they have evolved in such close alignment as to form a mutually-dependent human-microbe hybrid. Humans ingest an amount of food that they’re unable to digest but which nourishes the billions of microbes in their digestive tract. In return, these beneficial microorganisms extend human life expectancy by increasing absorption of important micronutrients, modulating stress and maintaining the balance of adipose tissue. They help humans to live longer and in so doing, ensure their own survival. The more useful and effective a person’s microbiota, the greater their chances of surviving – and therefore of reproducing – and the more likely they are to pass the same groups of bacteria on to their descendants. At birth, a mother’s gut flora is completely transferred to her baby. This unique and precious microbial mix ensures the new-born begins its independent life in the best possible health.
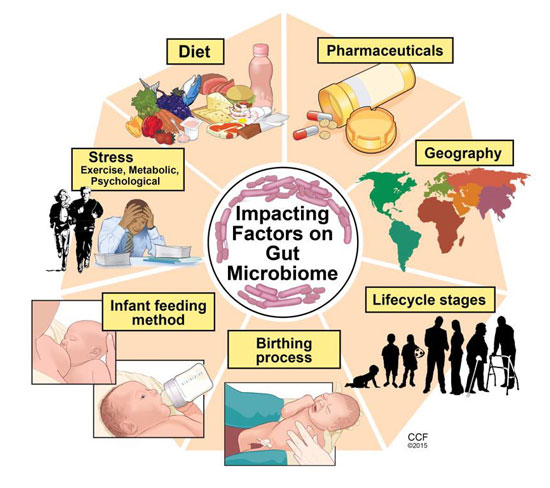
Over time humans have delegated more and more functions to this exceptional organ. Our microbiota is partly responsible for the maturation of our immune system: in teaching the immune system to respond in the right way to beneficial and pathogenic bacteria, it has improved its balance and helped our defences function optimally. It also modulates inflammation levels in the nervous system through its constant communication with the brain. We’ve put our trust in it without really realising it and now we can no longer do without it. But as modern lifestyles have become incompatible with our bodies’ needs, this is now a real problem. The fact that our diet has changed so dramatically means that we have in some ways ‘broken the contract’ with our microbiota. No longer able to find the right ‘fuel’ in the food we’re consuming, beneficial microbes are gradually deserting the gut. Worse still, we’ve added further obstacles in that the undigested elements of the questionable food we’re ingesting goes on to feed rival pathogenic bacteria! It’s hardly surprising then that things are in such a sorry state. When the microbiota suffers, so too does its human host: the result is increased sensitivity, instability and disease.
The 100,000 billion close friends we’re now losing touch with
The way our diet has evolved threatens the survival of these 100,000 billion ‘friends’ with which we’ve happily co-habited for thousands of years. And each of these friends is unique: there are no two identical microbiota in the whole world. At birth, we all have a particular combination of bacteria that depends both on our genes and on the strains we’ve inherited from our mothers, but this changes over time as a result of environmental factors, levels of physical activity and diet. In other words, we can never take the composition of our microbiota for granted: it has to be maintained as it can deteriorate at any time. And when that happens, beneficial microorganisms become less diverse, and bacteria not seen in a healthy microbiota appear. As a result of DNA sequencing, scientists have identified these kinds of bacteria in the digestive tracts of people suffering from diabetes, cirrhosis and obesity. It’s highly likely that the majority of these microbes play a role in weight gain and in the inflammation processes seen in numerous diseases.
"Auto-immune diseases and allergies are linked to gut dysbiosis” Georges Mouton author of ‘The gut ecosystem and optimal health’.
What would happen if these beneficial microorganisms were to abandon the gut altogether? Recent research has clearly shown that without these important friends, we mammals would not get very far. Tests were conducted on rodents raised ‘in a bubble’ - they had never come into contact with a single microbe (ie they had no microbiota). For these animals, nothing was easy: the absence of bacteria made them highly vulnerable to pathogenic germs and they rapidly fell victim to disease, allergies and stress. Just like humans, rodents have evolved symbiotically with a group of beneficial bacteria compatible with their needs and adapted to their specific diet. So the results of these tests can be easily applied to humans. With no gut microbiota, we could not expect to live a healthy life, or in all probability, live at all. Fortunately, we have not reached this point. For the moment, our microbiota is still alive, but its composition is changing and the quality of the strains of which it is comprised is deteriorating at an ever-increasing rate. The consequences of this are already evident and there’s every indication that this is just the beginning:
- The number and range of auto-immune diseases (type 1 diabetes, multiple sclerosis, rheumatoid arthritis, lupus, Reynaud’s syndrome …) is skyrocketing. These poorly-understood diseases are the result of a dysfunction in the immune system which makes it attack normal components of the body. They have been growing in number since the 1970s: almost 80 have been identified to date.
- Chronic inflammation, which is virtually systemic in auto-immune diseases, is also growing at a staggering rate. Often asymptomatic to start with, inflammation tends to become chronic once symptoms have appeared. Slowly but surely, it promotes changes in the organisation of cells and tissues and leads to the development of a wide range of diseases (cardiovascular, neurodegenerative, auto-immune …).
- The prevalence of allergies has risen massively over the last 30 years. Allergies are the consequence of irregularities in the immune system associated with lower tolerance to substances generally deemed to beskin problems (urticaria, dermatitis), respiratory problems (rhinitis, asthma) or more generally (anaphylaxia): it’s thought that an unprecedented 30% of the population are today affected by an allergic disease.
- Diseases affecting the cognitive system such as depression, schizophrenia and neurodegenerative disorders are also growing in prevalence and are directly linked to gut microflora.
The deterioration in our microbiota means that certain beneficial strains are being replaced by pathogenic ones, and that there’s a dramatic decline in microorganism diversity. Only a few years ago, it was thought that babies should be kept away from germs as much as possible. This was a time, not entirely relegated to the past, in which caesarean sections, intensive antibiotic therapy and the use of drastic hygiene measures all spiralled. We now know this to be a serious mistake: babies’ microbiota have been slow to diversify and adults today have defective immune systems that encourage the development of countless diseases. Scientific research has shown that mammals reared in a sterile environment develop more severe allergies than those reared in normal conditions, a finding consistent with earlier observations in which children growing up on farms (of which there are far fewer today) developed allergies to a much lower extent than those living in towns.
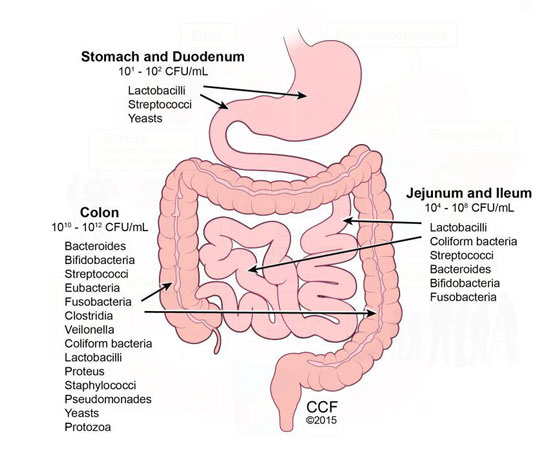
What’s the reason for this paradox? It’s during the first years of life that the immune system learns how to control itself. Microbes skilfully teach it how to recognise intruders, how to react appropriately, when to tolerate and when to resist. For its part, the immune system also learns to regulate its responses, to react less impulsively or more directly as the situation demands. It’s a real learning curve, at the end of which the body has learnt how to optimally balance its immune response. The greater the number and diversity of the bacteria, the better this ‘training’ will be. In today’s societies, these elements are unfortunately all too low, and our immune systems never achieve a sufficient degree of maturity.
When bacteria are poorly-nourished
Pet-owners worry more and more about the content of the kibble they feed to their faithful companions. Is it really suitable for their needs? What proportion of cereals does it contain? Does the amount of micronutrients it offers correspond to what they would eat in nature? These are legitimate worries, especially when we know the abuses of the agro-food industry destined for human consumption. At the same time, these concerns are somewhat incongruous given that these same people give absolutely no thought to the diet needed by organisms much closer to them – those living in their gut.
Yet like these individuals’ four-legged friends, their symbiotic microbes also have dietary preferences. The vast majority of them feed on dietary fibre, the long polysaccharide chains found in abundance in fresh fruit and vegetables and wholegrains. Each species has its own enzymes for breaking down, fermenting and using the energy contained within the fibre which the human body is unable to digest. In a healthy microbiota, this fibre is broken down into increasingly tiny fragments, from the small intestine to the bowel, before disappearing completely at the end of the chain. Remove it from your diet, and these bacteria will gradually abandon your gut environment, to your great disadvantage. Significantly increase your fibre intake (making sure you do so gradually) and conversely, you’ll expand the diversity of these beneficial bacteria.
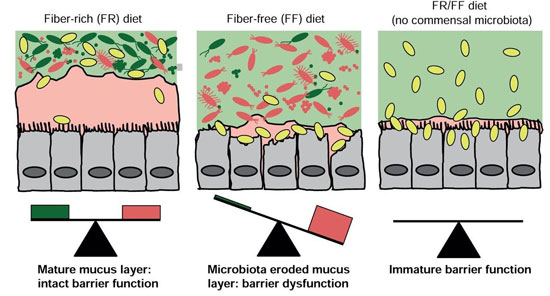
Unfortunately, the fibre content of modern diets tends to be decreasing rather than increasing. The decline in culinary traditions, the industrialisation of food, the refining of cereals, the lack of fresh plant-based foods: all these factors combine to reduce fibre consumption and pose a serious threat to the microbiota. And we make things even worse by encouraging rival pathogenic microorganisms.
The bad company we’re now keeping: pathogenic bacteria and fungi
Our bodies’ frequent exposure to antibiotics, whether as a result of regular medication, or indirectly through consumption of farm-raised animals, seriously disrupts the microbiota. By definition, these drugs impede bacterial function: thus in the medium-term, they lead to long-lasting and sometimes permanent eradication of bacteria populating the digestive tract. When antibiotic treatment ends, some bacteria will of course repopulate this vacated environment, but not all beneficial bacteria find their way back...
There are other environmental factors that promote competing pathogens. With the exception of athletes and the elderly, most people consume far too much protein (consumption in Europe is around 1.7 times higher than is recommended), as a result of almost unlimited access to animal-source products. Some of this excess is neither digested nor absorbed by the body: it is therefore still intact when it reaches the colon and comes into contact with the microbiota. To some extent, intestinal bacteria manage to break it down but as they do so, two by-products toxic to gut mucosa cells are created: hydrogen sulphide and p-cresol. These two compounds are able to enter the bloodstream and induce inflammation in a number of organs such as the liver and kidneys. In consuming excessive levels of protein, we are encouraging those bacteria with an affinity to it, and stabbing our longstanding friendly bacteria in the back. The same thing also happens when we consume completely inappropriate amounts of refined sugar - which we do almost every day. This transformation of our diet has serious consequences since mothers then pass on their microbiota to their babies, and so with each generation, the quality of microflora deteriorates (20).
How can we minimise our contact with these undesirable bedfellows that promote chronic inflammation and disease? As is often the case with food, it’s not easy: with no warning, a vicious circle is quietly taking hold. Researchers have discovered that our instincts can be influenced by the nature of the microorganisms populating our digestive tract! In other words, the billions of bacteria feeding on the remains of our meals may play a role in dictating what food we crave. If your gut flora is poor, adapted to a diet excessively high in animal protein for example, you may be more readily disposed towards a meat-rich menu simply because your microbiota is influencing you to do so. This fascinating research opens up new prospects for improving our understanding of problematic food behaviour...
The amazing power of fibre
The lack of maturity in our immune systems cannot now be put right. But there is still time to ensure we maintain or restore the healthiest microbiota possible. There are two major routes to doing so. The first, as we’ve already mentioned, centres on gradually increasing our dietary fibre intake (also referred to as prebiotics), while reducing that of animal protein and refined sugar. In this way, we promote the development of healthy bacteria and starve those that are pathogenic. It is relatively easy to obtain dietary fibre as it’s found in practically all pulses and fresh fruit and vegetables. However, for those who find it difficult to eat more than 10 portions of fruit and vegetables a day (due to time constraints, for example), there are natural-source prebiotic supplements such as fructo-oligosaccharides, derived from beetroot and in a sufficiently-concentrated form to feed millions of beneficial bacteria.
Adopting this approach makes it easy to recolonise the gut with beneficial bacteria, particularly those that release short chain fatty acids (butyrate and propionate). These relatively unknown compounds are to a large degree responsible for the beneficial properties attributed to the microbiota. As the following list illustrates, they have far-reaching effects on our health:
- Butyrate acts on the permeability of the gut mucosa. It strengthens adhesion and solidity in the junctions between the cells of the gut wall by increasing the expression of several structural proteins. As a result, it reduces the passage of bacteria and certain compounds (toxic lipopolysaccharides and antigens widely-found in processed food) across the wall, which is responsible for chronic inflammation.
- Propionate and butyrate reduce the production of lipids by hepatocytes in the liver and prevent Kupffer cells from generating pro-inflammatory cytokines.
- Short-chain fatty acids facilitate satietythe development of diabetes).
- Propionate and butyrate interfere with immune cells to reduce the intensity and incidence of inflammatory diseases.
In the light of these remarkable properties, you may wonder why it is we don’t simply consume short-chain fatty acids directly. The fact is they’re only present in tiny quantities in foods such as butter and cheese, the liberal consumption of which is not necessarily recommended. So it’s better to focus on achieving a stable gut flora that’s able to produce a continuous supply of these compounds, close to their sites of action.
Remember: daily consumption of 60-80 grams of fibre will, in just a few months, significantly increase the diversity of microbiota and enrich it with bacteria able to release propionate and butyrate.
Probiotics: a medicine of the future – available today
The second solution is based on pioneering work highlighting the immense therapeutic power of probiotics. These microorganisms, often bacteria, come from foods such as dairy yogurt and kefir, and improve gut flora. Their effects are temporary: they do not establish themselves as commensal bacteria of the human microbiota but they do encourage the return of such microbes, and at the same time, repel intruders.
The notion that bacteria from certain foods are beneficial to health is, however, nothing new! As long ago as 1908, the scholar Ilya Metchnikov was already suggesting that the astonishing longevity enjoyed by Bulgarians was due to their high yogurt consumption. At the beginning of the century, this type of product was actually only sold in pharmacies … Since then, scientists have made huge progress, conducting thousands of studies on probiotics and the microbiota. We now know that the brain and microbiota are in constant communication with each other, and that this dialogue is bilateral: the brain sends messages to symbiotic microorganisms and vice versa, and these messages affect a wide variety of physiological and psychological mechanisms (anxiety, depression, mood disorders, emotional responses …).So how do probiotics act to improve these remarkable exchanges? There are three distinct mechanisms:
- In young children, they promote healthy maturation of the child’s immune system, and so discourage allergic reactions.
- In adults, they help stimulate certain beneficial bacteria which produce butyrate, a fatty acid with exceptional properties.
- Through competition for resources, they reduce populations of certain pathogenic bacteria, particularly those such as Bilophila wadsworthia thought to be involved in the onset of inflammatory and intestinal diseases.
The latest discoveries touch upon some quite astonishing associations between our microbiota and aspects of our health ….
1) Neuro-inflammation
The ingestion of probiotics such as the lactic bacteria Lactobacillus farcimins may help make the intestinal barrier less permeable, and so reduce the extent to which pro-inflammatory compounds are able to enter the bloodstream. Such compounds are implicated in the development of neuro-inflammation in the brain, exacerbating the adverse effects of stress and significantly increasing the risk of neurodegenerative diseases. Neuro-inflammation may even be directly linked to cognitive decline!
A recent study published in September 2018 in Frontiers in Immunology
“What you eat is important. We know that seniors consume 40% less fibre than is recommended. Not eating enough fibre can have negative consequences in ways you wouldn’t imagine, such as on brain health and inflammation in general”, Jeff Woods, Professor of Kinesiology and co-author of the study.
2) Dermatological problems
We’ve known for a number of years that there’s an association between pro-inflammatory compounds entering the bloodstream and inflammation of the skin, but an entirely different explanation has been suggested for the negative dermatological effects of an unhealthy microbiota. Probiotics and beneficial bacteria control the immune system by activating several types of cell, such as dendritic cells, T lymphocytes and NK cells. These cells, which are abnormally reduced in animals deprived of gut flora, help increase production of interleukin 10 (IL10) (9-10), a cytokine with significant anti-inflammatory power which is secreted by the body in response to a number of conditions such as eczema and dermatitis. Researchers have wondered about the connection between the microbiota and skin disorders since intestinal transit problems and diseases such as Crohn’s and IBS were observed to often manifest in additional dermatological problems.
It is on the basis of these very recent studies that new probiotics have been introduced over the last few months. These include Derma Relief, a formulation containing four strains of probiotic chosen for their benefits for the skin (Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum and Bifidobacterium lactis). Studied by Dr Fabio Meneghin as part of a review of 37 clinical trials into the dermatological benefits of oral probiotics, these gut-colonising strains were shown to be effective both preventively and therapeutically!
The gut revolution is underway
With the recent sequencing of the human genome and the identification of genetic predisposition, we have already taken a giant step towards the medicines of the future. All that remains is to fully understand the fascinating community that is the microbiota and to produce tailor-made human bacteria to prevent and treat all the diseases mentioned in this article. The challenge will then be to mechanise production of these bacteria and produce them in powder form, as they are highly sensitive to oxidation. Progress has already been achieved by A-Mansia, a biotech spin-off established by two universities, with the development of a dietary supplement based on Akkermansia, one of the many species of bacteria that make up our gut microbiota. While we await results from the first clinical trials, we’ll need to continue to rely on dietary probiotics (there’s a list at the end of this article), the benefits of which appear to be ever more numerous. They represent the dawn of a type of medicine which finally takes account of the fact that humans and their microbiota are a symbiosis essential for maintaining health and well-being. A genuine revolution is emerging...
Particular individuals who should re-read this article:
- those who don’t eat enough fruit and vegetables;
- those who eat a lot of meat (at least once a day on average);
- those who suffer from allergies or auto-immune diseases;
- those with eczema or other skin problems;
- those who smoke or who are exposed to secondary smoke on a daily basis;
- those who are often under stress;
- those with frequent or chronic gut problems;
- those who regularly eat ultra-processed food;
- those over 50 and who are worried about the onset of cognitive decline.
List of probiotics
For gut problems: Lactobacillus gasseri
For inflammatory problems and allergies: Probio Forte™ (Lactobacillus acidophilus, Lactobacillus casei , Lactobacillus plantarum, Lactobacillus lactis)
For dermatological problems : Derma Relief (Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Bifidobacterium lactis)
The key points to take away from this article:
- The microorganisms that make up our gut flora play a far more important role in our health than was previously thought.
- The diversity of these microbes is in sharp decline because of the dramatic deterioration in our diet.
- Adding more dietary fibre to the diet and taking probiotics are two successful strategies for restoring the diversity and efficacy of the microbiota.
References
Stephanie M. Matt, Jacob M. Allen, Marcus A. Lawson, Lucy J. Mailing, Jeffrey A. Woods, Rodney W. Johnson. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Frontiers in Immunology, 2018; 9 DOI: 10.3389/fimmu.2018.01832Douglas J. Morrison & Tom Preston (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism, Gut Microbes, 7:3, 189-200, DOI: 10.1080/19490976.2015.1134082
Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 2015; 74:13-22; PMID:25268552; http:// dx.doi.org/10.1017/S0029665114001463
INRA Science & Impact, Microbiote, la révolution intestinale, 2017 [https://inra-dam-front-resources-cdn.brainsonic.com/ressources/afile/383885-34a7b-resource-dossier-de-presse-microbiote-la-revolution-intestinale.pdf]
Adrián D. Friedrich, Mariela L. Paz et al. Message in a Bottle: Dialog between Intestine and Skin Modulated by Probiotics, Int J Mol Sci. 2017 Jun; 18(6): 1067.
Agusti A. Garcia-Pardo MP et al. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function, Front Neurosci. 2018; 12: 155. doi: 10.3389/fnins.2018.00155
 Fructo-Oligosaccharides
Fructo-OligosaccharidesBifido-active fibre. Improves intestinal health and boosts the body's own natural defences
www.supersmart.com Lactobacillus Gasseri
Lactobacillus GasseriA probiotic strain that’s particularly effective for weight control
www.supersmart.com Probio Forte
Probio ForteProbiotic mix; 8 billion microorganisms per capsule. In gastroresistant DR Caps™ for optimum efficacy
www.supersmart.comAll rights reserved
Free
Thank you for visiting our site. Before you go
REGISTER WITHClub SuperSmart
of exclusive benefits:
- Free: our weekly science-based newsletter "Nutranews"
- Special offers for club members only